Prostate Cancer: Integrating Newer Imaging Modalities Into Clinical Practice
Interesting questions raised by a paper that compared the use of 18F-fluciclovine with 68Ga-PSMA-11 imaging modalities to detect biochemical relapse in prostate cancer and implications for integrating newer techniques into clinical practice.
Episodes in this series
Steven Finkelstein, MD, DABR, FACRO: One interesting question raised is, is the same going to hold true in gallium PSMA [68Ga-PSMA-11] as it is a PYL [18F-DCFPyL]? Obviously, PYL is going to be a lot more available to the general community. Louis, you can comment on that a little.
Louis J. Mazzarelli, MD: The literature so far when you look at CONDOR and some of the comparative studies with gallium-68 shows that these agents perform very similarly, whether they’re F-18 [18F-fluciclovine] or gallium-68 based. That’s 1 thing that’s been shown. Another question is, what does this paper tell us about disease heterogeneity within the world of prostate cancer? What are the potential implications that that heterogeneity may have for treatment of disease? Whether that’s small molecule-based theragnostics or the ability to identify low-volume disease, mono-metastatic or oligometastatic disease. We’re heading toward a world of theragnostics where we’re going to be able to use small-molecule targets to identify small volume of disease by a PSMA [prostate-specific membrane antigen] study because most agents being developed are PSMA based. How does that affect a patient who has a 18F-fluciclovine study that has positive lesions that the PSMA study doesn’t pick up? I believe these patients have some degree of heterozygosity. How that’s impactful for the patient is another question.
Steven Finkelstein, MD, DABR, FACRO: That’s excellent. For me, I find the same. As a radiation oncologist, the theragnostics are exciting. We’re able to take images where we see exactly where there’s evidence of cancer recurrence and provide the right personalized therapy that’s targeting those sites of disease using radiation modalities. [That’s regardless] whether it’s what I do as a focused radiation oncologist using linear accelerator-based approaches, or I’m doing theragnostic work, giving target agents as well as our nuclear medicine colleagues. One of the most important steps coming down the pike is the PYL part of the story, the fact that 68Ga-PSMA-11 scans are hard to get and the PYL scans are going to be much more available is a really exciting thing about how to incorporate that in the community patient.
The other thing that’s exciting for next steps is that PYL–AI [artificial intelligence] is approved to help readers detect and read these scans in a more reproducible way. There’s some belief that this could correct some of the hot bladder issues with respect to imaging, and that’s going to be a very exciting part of the story as it comes up.
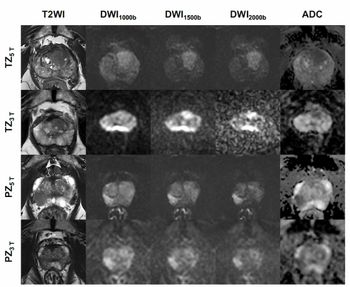
As we land the plane and get to our conclusions, with respect to this study, the advantage of F18-fluciclovine was in detecting curable localized disease in close anatomic relation to the urinary bladder. Whereas PSMA PET [positron emission tomography]—in this case, 68Gallium-PSMA-11—fails because of accumulation of activity in the urinary bladder. The modalities are equivalent in detecting distant metastases, although you can see the advantage in most situations for using PSMA PET when we’re talking about the detection of distant sites of disease. Louis, I’m going to let you finish and get the last word.
Louis J. Mazzarelli, MD: From an imaging perspective, this is a new era. This is certainly a new world. When we look at where we were with conventional imaging techniques [and compare it with] where we are [and look] into the future of theragnostics and localized therapy for small-volume disease, we have new tools that are redefining how we care for patients with prostate cancer and the whole prostate cancer algorithm. We’re very lucky in many ways to have 2 outstanding radiotracers that allow us to identify disease. Both potentially have utility in the future, even in the world of tumoral heterozygosity or 1 agent being more focused toward local recurrent, the other 1 more distance disease. We’re still defining those features. We still have work to do with respect to PSA [prostate-specific antigen] demographics. We need to subcategorize patients into their PSA quartiles but also the subtypes of Gleason tumors that they have. It’s important because these tumors will perform differently. But equally, we have a horizon that’s going to save a lot of people’s lives. It’s going to be very impactful in the future for how we treat these patients.
Steven Finkelstein, MD, DABR, FACRO: With that, on behalf of Louis, thank you to the CancerNetwork® [for allowing us to] go over this exciting work today. It’s an exciting time to be a cancer doctor trying to help patients with prostate cancer. I hope you had a good educational experience, and we look forward to another CancerNetwork® presentation.
Louis J. Mazzarelli, MD: Many thanks. Good evening, everyone.
Transcript edited for clarity.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.