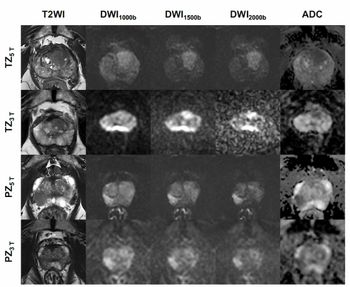
Background: 18F-Fluciclovine and 68Ga-PSMA-11 Imaging in Prostate Cancer
Drs Steven Finkelstein and Louis J. Mazzarelli comment on limitations associated with conventional imaging modalities in prostate cancer and highlight the roles of newer tests such as 18F-fluciclovine and 68Ga-PSMA-11.
Episodes in this series
Steven Finkelstein, MD, DABR, FACRO: Hi, my name is Dr. Steven Eric Finkelstein. I’m a radiation oncologist at Florida Cancer Affiliates, the US Oncology Network [in Panama City, Florida]. I’m here with Louis Mazzarelli to present this CancerNetwork® featured discussion, “A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT.”
Many of those on the webinar probably know me. I’m a radiation oncologist in Florida. I was a former surgeon at the National Cancer Institute and found that radiation were radiation imaging was interesting. I went back to school, did a radiation oncology residency and a chief residency at [H. Lee] Moffitt Cancer Center [in Tampa, Florida]. Now I work for the US Oncology Network. I’m going to have Louis introduce himself.
Louis J. Mazzarelli, MD: Good evening, everybody. It’s a pleasure to be here. Steve, it’s a pleasure to meet you. My name is Dr Louis Mazzarelli. I’m a diagnostic radiologist at Lawrence + Memorial Hospital, which is part of the Yale New Haven Health network [in New London, Connecticut]. I have a passion for prostate imaging, both on the molecular imaging side and also on MRI, so this will be particularly fascinating from that perspective. I’m looking forward to talking about it.
Steven Finkelstein, MD, DABR, FACRO: Let’s get into the paper. This was a prospective head-to-head comparison of 2 exciting imagings in the recurrent setting for prostate cancer. A little background as we think about this: 20% to 40% of patients with prostate cancer will experience a disease recurrence in their lifetime. Recurrence is usually diagnosed based on a rising PSA [prostate-specific antigen]. When you have a recurrence, some common sites after a total radical prostatectomy include the vesicourethral anastomosis, the urinary bladder at the neck, and the rectovesical space. For what I do as a radiation oncologist, a local recurrence can occur at the site of the primary tumor—ie, in the prostate. However, the accurate detection of prostate cancer remains a challenge. Classically we haven’t had the best imaging available. Louis, what do you think about what we’ve had for imaging before we get into some of the next-generation imaging?
Louis J. Mazzarelli, MD: Conventional imaging had many limitations with respect to the ability to identify recurrent prostate TA [transit-amplifying] cells. Not uncommonly over the last 15 years that I’ve been practicing, before the advent of incredibly exciting molecular imaging techniques, we were asked to identify prostate recurrence in patients with low or even high PSAs, and we weren’t able to do that with conventional imaging. We certainly weren’t able to see the anatomy locally, whether at the vesicourethral anastomosis. Certainly we weren’t able to identify the prostate well, and we’re talking from CT perspective. Of course, the advent of MRI allowed a change especially for local evaluation of prostate. But when it came to the analysis of metastatic disease or local nodal disease or extrapelvic nodal disease, we were very limited. An MRI has significant limitations from the basis of identifying nodal disease within the pelvis and extrapelvic spaces. This was a huge change with the advent of these 2 radiotracers. Of course, 18F-fluciclovine was the first, and now PSMA [prostate-specific membrane antigen] is available. It’s an exciting time.
Steven Finkelstein, MD, DABR, FACRO: This paper goes into a head-to-head comparison of these 2, what we would call next-generation imaging modalities. Louis, talk a little about Axumin, 18f-Fluciclovine. Why don’t you start by about your experience with it?
Louis J. Mazzarelli, MD: That was our first next-level imaging device, imaging apparatus, imaging tool. It was a great starting point. It’s an amino acid–based imaging tool. What it allowed us to do was look for recurrence. When it was approved, it was approved for recurrence and allowed us to look at the beds, nodes, and bony disease. We’ll get into this as we look at the paper itself, but in terms of the physiology, PSMA and Axumin, or 18f-Fluciclovine, are different in the sense that PSMA has a PSMA-directed targeting membrane, whereas Axumin [18F-fluciclovine] is an amino acid–based compound. Therefore, the physiology in the body is going to be different. When we look at Axumin [18F-fluciclovine], we look at background uptake in muscle and bone, and we look at much less genitourinary or urinary excretion. When we look at PSMA we look at an agent that has more urinary excretion but is more sensitive with respect to uptake within bone and soft tissues. They’re different radiotracers, and we’ll see a couple of images that we’ve selected from the paper.
Steven Finkelstein, MD, DABR, FACRO: Perfect. My experience with Axumin [18F-fluciclovine] as a radiation oncologist was in a world where we really didn’t have a way to image and find sites of recurrence. We’d have people with rising PSAs, and you couldn’t find with the conventional imaging modalities. We’d do technetium-99 bone scans. We wouldn’t find anything. Then we have the ability to do Axumin [18F-fluciclovine], and if I’m right, it was an imaging modality that was originally for something else. I believe it was for cardiac imaging when General Electric originally had it. Is that correct, Louis?
Louis J. Mazzarelli, MD: The beginnings of it are—I’m not as certain about that. I know that when it was developed at Emory [University], it wasn’t necessarily developed for this purpose. I’m sure that history is true. But it was found to be a nice bridge. Before this we had choline as an adjuvant for evaluation from a molecular imaging perspective. The issue with choline of course is its short half-life and needing an on-site cyclotron. Not many places are able to generate choline. So when 18F-fluciclovine was put in the market as a fluorine-based molecular imaging agent, its availability increased dramatically. And you’re absolutely right: bone scans have a degree of sensitivity, but they require there to be enough blastic activity within the bone to see the disease. Unfortunately, that has sensitivity and specificity issues. When Axumin [18F-fluciclovine] came on the market, we were excited because we had an opportunity to look locally and distant, both specifically in the nodes and for bone metastatic. It was a huge change. Now there are new indications for using 18F-fluciclovine, including in the brain. It’s a versatile radioactive tracer.
Steven Finkelstein, MD, DABR, FACRO: That’s fantastic. With that, let’s transition a little to the PSMA, the gallium PSMA [68Ga-PSMA-11] PET [positron emission tomography]. In the United States, 2 places on the West Coast have access to 68Ga-PSMA-11. Many in the United States who are watching this probably don’t have yet access to gallium-68. Maybe you could talk a little about this agent, 68Ga-PSMA-11, and talk about your experience. If you haven’t used it per se, talk about what you’ve heard from other people using it. We’ll go from there.
Louis J. Mazzarelli, MD: Initially, when gallium-68 was put on the market, it was available only on the West Coast: UCSF [University of California San Francisco] and UCLA [University of California, Los Angeles]. Most institutions on the East Coast and the middle of the country were not able to have access to it. What changed was the ability of imaging sites to have access to F18 PYL [18F-DCFPyL], which is a PSMA agent that’s bound to F18 as opposed to gallium-68. So gallium-68 needs to be prepared and chelated as opposed to F18 PYL, which is produced through standard PET mechanisms and is more readily available and distributed. They are both PSMA-target antigens, so they function identically. What’s different is the amount you can make of gallium-68 vs the amount you can make of PYL or other developing F18-fluciclovine–based PSMA agents. They differ a little in how the images look. Some people will say that the F18 is a little cleaner than the gallium-68. But functionally, all the studies suggest that their performance is very similar in terms of the ability to identify recurrent disease. It’s a matter of where you are and what you have access to.
Transcript edited for clarity.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.