
Catch up on the top AI-related news and research in radiology over the past month.

Catch up on the top AI-related news and research in radiology over the past month.
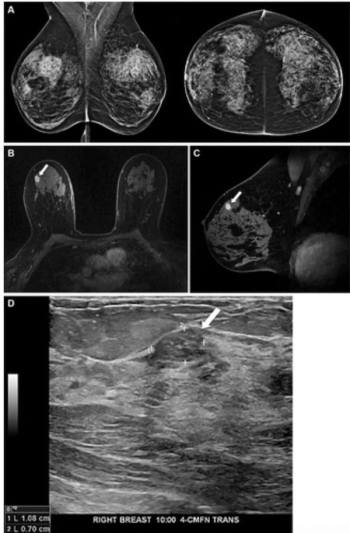
New research suggests the capability of a mammography-based deep learning model to identify women at high risk of breast cancer led to more than triple the cancer detection rate on breast MRI in comparison to traditional risk assessment tools.
The artificial intelligence (AI)-enabled AIRAscore can reportedly provide quantitative brain volume data within five minutes of assessing brain MRI scans.

Catch up on the top radiology content of the past week.
In a recent video interview, Vivek Bansal, M.D., discussed a recent Radiology Partners publication that offers practical tips and best practice guidance on CT and MRI neuroimaging for stroke patients.
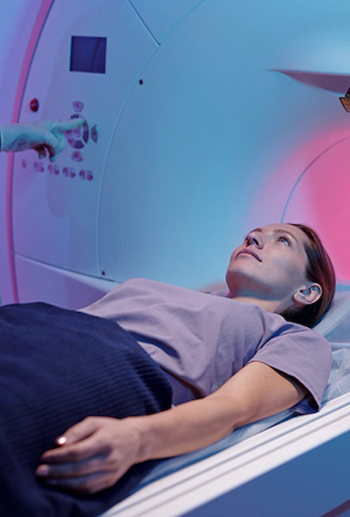
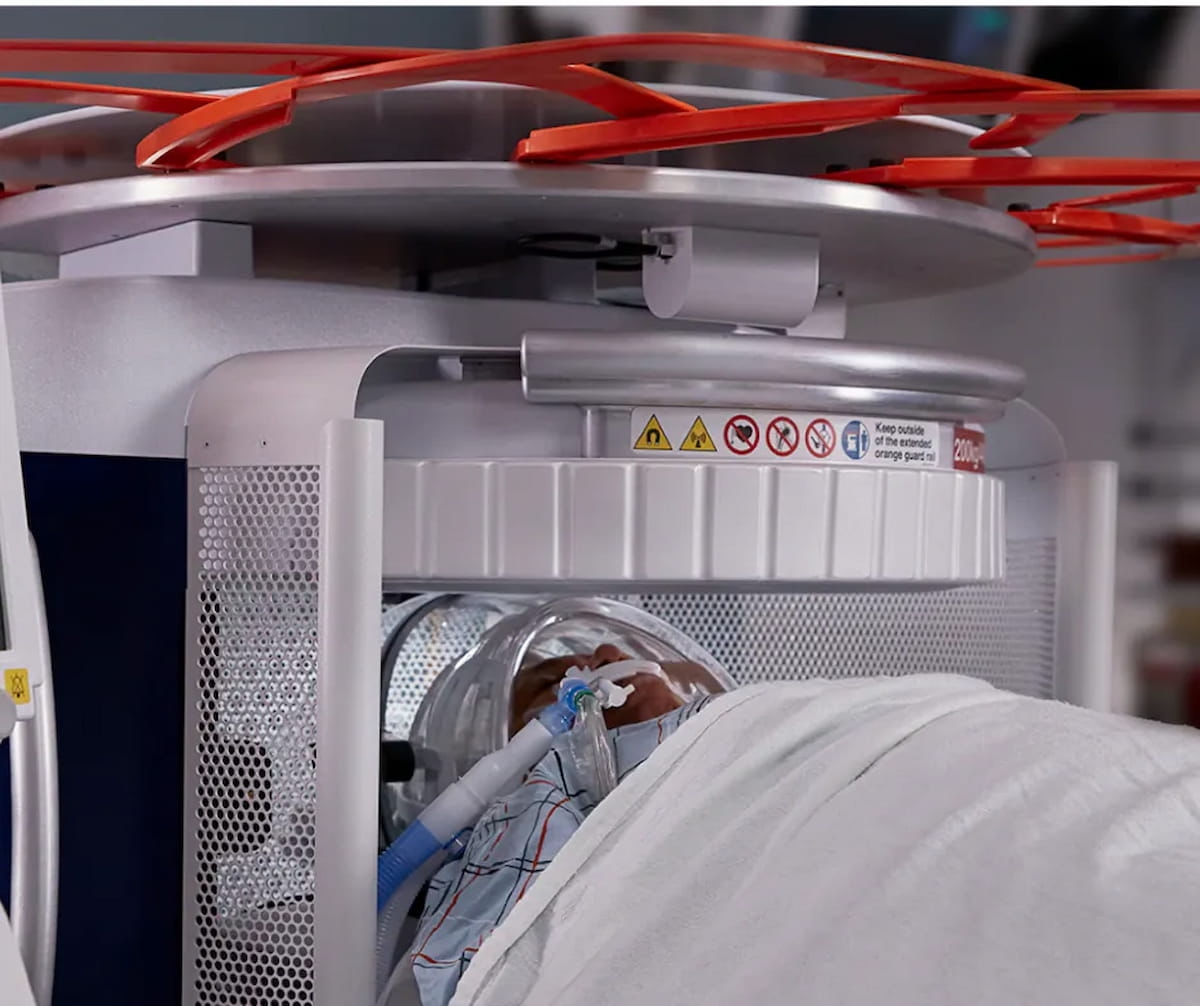
A robotic guidance and placement system, the IGAR system can be utilized inside of a magnetic resonance imaging (MRI) bore.

Catch up on the top radiology content of the past week.
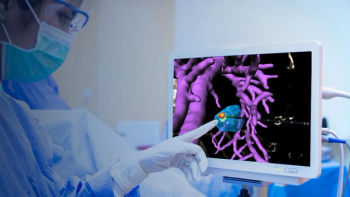
Through the use of artificial intelligence (AI) and imaging modalities such as ultrasound, CT, and MRI, the newly FDA-cleared VisAble.IO software reportedly enhances planning and real-time assessment for liver tumor ablation procedures.

Catch up on the top radiology content of the past week.

Catch up on the top five most viewed content at Diagnostic Imaging in August 2023.

Catch up on the top AI-related news and research from the past month.

Catch up on the top radiology content of the past week.
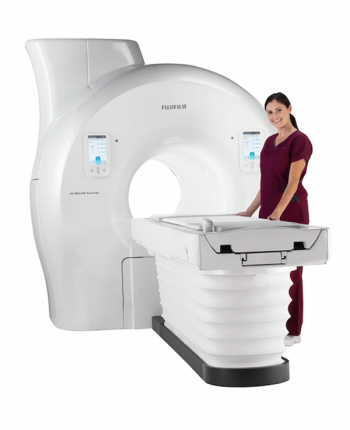
The Echelon Synergy MRI system reportedly uses deep learning technology to accelerate image acquisition and enhance image quality.

Catch up on the top radiology content of the past week.
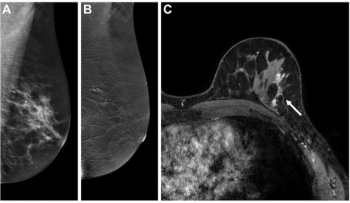
In a new study comparing standard breast MRI, abbreviated breast MRI and contrast-enhanced mammography in supplemental breast cancer screening, researchers found that MRI offered a greater than 14 percent higher cancer detection rate and a nearly 39 percent higher sensitivity rate than CEM.

Catch up on the top radiology content of the past week.
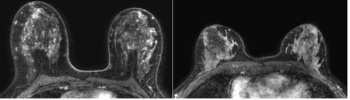
Emerging research suggests that a high volume of enhancing parenchyma on dynamic contrast-enhanced MRI more than doubles the breast cancer risk for women with extremely dense breasts.
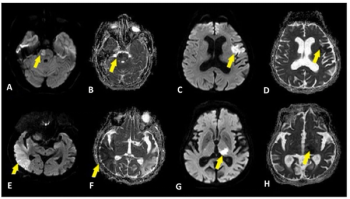
The most common brain MRI findings associated with COVID-19 included acute/subacute infarction in 22 percent of patients and cerebral microbleeds in 17 percent of patients.
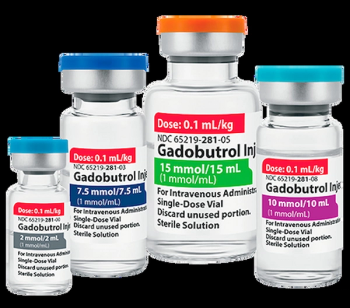
The indications for Gadobutrol injection, a generic substitute for Gadavist, include neurovascular and cardiovascular assessments as well as breast malignancy detection with magnetic resonance imaging (MRI).

Catch up on the top AI-related news and research from the past month.
What do you do with a CD of imaging that has no information on the doc requesting the radiologist read?
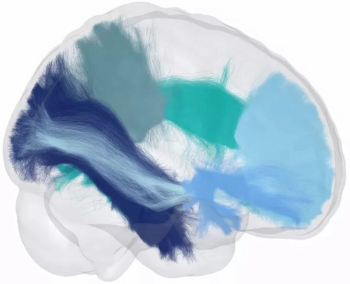
Through automated processing of diffusion-weighted magnetic resonance imaging (MRI) scans, the Advanced Neuro Diagnostic Imaging (ANDI) software performs detailed analysis of white matter microstructure.

Catch up on the top radiology news of the past week.
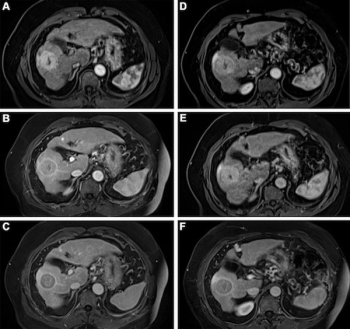
Newly published research suggests the use of gadopiclenol at 0.05 mmol/kg is non-inferior to gadobutrol 0.1 mmol/kg for all qualitative visualization parameters on full-body magnetic resonance imaging (MRI).
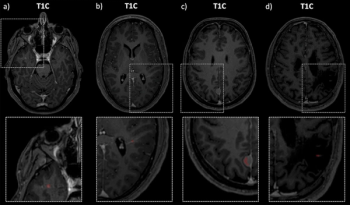
An artificial intelligence model, trained on MRI and FLAIR imaging from over 900 patients with multiple sclerosis, demonstrated a 96 percent accuracy rate and 99 percent specificity rate for contrast-enhancing lesions in this patient population.