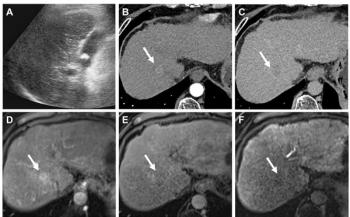
FDA warning flummoxes ultrasound contrast trial
Plans for a clinical trial designed to overcome long-standing regulatory obstacles to the general clinical use of ultrasound contrast media in the U.S. have been knocked off track by an FDA-mandated black box safety warning for two microbubble agents approved for echocardiography.
Plans for a clinical trial designed to overcome long-standing regulatory obstacles to the general clinical use of ultrasound contrast media in the U.S. have been knocked off track by an FDA-mandated black box safety warning for two microbubble agents approved for echocardiography.
The black box warning for Bristol-Myers Squibb's Definity and GE Healthcare's Optison agents-issued Oct. 15-emphasizes the risk of serious cardiopulmonary reactions.
FDA representatives in 2006 had expressed general support for a draft plan from the American Institute of Ultrasound in Medicine for a clinical trial testing the efficacy of microbubble contrast for imaging transarterial chemoembolism of liver lesions. FDA officials suggested that the AIUM group use an approved cardiac agent (Definity or Optison) in its trial, according to Dr. Lennard Greenbaum, immediate past president of the AIUM.
"The black box ruling came as a total surprise," Greenbaum told Diagnostic Imaging. "At stake is whether ultrasound contrast agents will ever be used for a noncardiac indication."
The black box order followed reports of 11 deaths associated with the two agents. According to the order, four of those deaths were caused by cardiac arrest that occurred either during infusion or within 30 minutes after infusion of a microbubble ultrasound agent. FDA officials acknowledged during a November teleconference a lack of corresponding adverse reactions to microbubble contrast when administered for noncardiac indications.
Still, FDA officials turned down a request during the teleconference to waive its requirement for the black box warnings on microbubble contrast media to be used in the proposed AIUM trial. It also held to a rule requiring close patient monitoring in the 30 minutes following contrast administration.
"Some radiologists tell me there may be difficulty getting an institutional review board to approve a project associated with such a severe warning," Greenbaum said.
Dr. Michael L. Main, medical director of the electrocardiography lab at the Mid America Heart Institute, along with 160 cosigners, sent a letter in November to the FDA expressing concern about the black box warning. Main argued that the four patient deaths may have been caused by underlying cardiovascular disease. He also said that the deaths occurred over a six-year period while about two million doses of the ultrasound contrast media were administered. The data suggest an event rate for ultrasound microbubble contrast of one in 500,000 cases, which Main said compares well against the one in 2500 risk of cardiac death from exercise treadmill testing.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.