Cedara Software prepares to market CAD package for breast ultrasound
Cedara Software has submitted an application to the FDA to market software that promises to help physicians differentiate benign from malignant lesions on breast sonograms.
Cedara Software has submitted an application to the FDA to market software that promises to help physicians differentiate benign from malignant lesions on breast sonograms.
The Canadian software developer is touting B-CAD's potential to establish a new standard of care in women's health. The technology will improve the diagnostic contribution of ultrasound so much that fewer biopsies will be necessary, according to the company.
The software was created by Medipattern, a start-up company in Toronto. It addresses 40 characteristics of breast lesions cited by the American College of Radiology for their relevance in the diagnosis of breast cancer. The algorithms are based on research conducted a decade ago by Dr. A. Thomas Stavros and colleagues who developed a database of correlations between sonographic findings and biopsies.
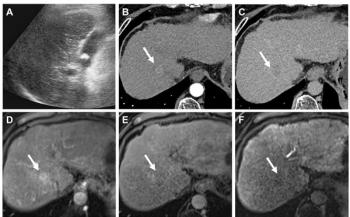
B-CAD interprets ultrasound images obtained on individual patients, then calculates the probability that a target lesion is cancer. This probability is then interpreted by the physician in the context of other findings, including those obtained during routine sonography and mammography.
The software has the potential to reduce variability in breast ultrasound, according to Cedara. This should provide physicians with a better foundation for determining the BI-RADS (Breast Imaging Reporting and Data System) category. BI-RADS 1, for example, means there is no sign of cancer, BI-RADS 3 identifies the abnormality as probably benign, and BI-RADS 5 indicates an abnormality highly suggestive of cancer.
Cedara considers B-CAD a global product. It was shown as a work-in-progress at the RSNA meeting and again at the European Congress of Radiology.
Despite its name, however, B-CAD is not a computer-aided diagnostic tool, at least not in the strictest sense. The software package is a sophisticated and advanced system developed for interpreting breast sonograms. But rather than rendering a stand-alone opinion or second reading, which is the approach of some CAD programs, B-CAD involves the physician at critical steps in its analysis. When outlining the lesion, for example, B-CAD defers to the physician to choose a "best fit" from several possibilities.
The reason for involving the physician was more political than clinical, according to Sabrina Cannistraro, Cedara's product manager for B-CAD. Pure CAD must meet a higher regulatory standard than less automated software. For example, mammography CAD, which is highly automated, requires clinical testing. FDA review of these systems can take a year or longer.
Software company Confirma learned, however, that the regulatory process goes much faster if the software is interactive. Its CADstream product keeps radiologists in the loop, while automating many of the time-consuming tasks necessary to analyze MR mammograms (SCAN 4/2/03).
Cedara worked with the regulatory consultant who hatched the strategy behind CADstream, drawing ideas from her experience. Cedara relayed its advice last year to Medipattern, where engineers scaled back the automation to make the physician an integral part of the analysis.
"This was really exciting for us because it meant we could be able to bring this product to market in six months rather than two or three years," Cannistraro said.
The work-in-progress software will likely be priced between $35,000 and $40,000, she said. Exactly how B-CAD will be applied in clinical practice has yet to be determined. The company has several options. One is to run the software on a desk- or laptop computer, which could be located in the same room as the ultrasound equipment. Another is to sell or license the software for integration into the ultrasound equipment itself. The third is to make the package available as a module that can be plugged into a PACS or workstation.
One candidate for the latter option is Cedara's I-ReadMammo, a software-based workstation designed to serve as a one-stop center for clinical workflow in breast imaging. It can simultaneously display breast images from several modalities, as well as present the results from computer-aided diagnostics. B-CAD was shown at the RSNA meeting running on this workstation.
Steps to market B-CAD in the U.S. will have to wait on the FDA. If the agency accepts the submission and delivers a decision in its usual 90-day window, Cedara could be selling B-CAD in early summer.
While North America is the primary market, it is not the only one, which is the reason Cedara showcased the technology at the ECR in Vienna. Cannistraro noted that Cedara's ability to distribute this product worldwide was a principal reason Medipattern decided to partner with a fellow Canadian company. Since then, prospects for distribution have grown.
Cedara's ability to distribute B-CAD as a component of PACS increased last year with the company's acquisition of PACS vendor eMed Technologies. It could grow again, if Merge eFilm, another PACS vendor, succeeds in its bid to acquire Cedara.
Sometimes proposed mergers can have a chilling effect on the development of new products, as they raise uncertainties about the future. Such is not the case this time, however, according to Cannistraro.
"It's full steam ahead on this one," she said.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.