Molecular Imaging
Latest News

Study Shows No Impact of Hormone Therapy on PET/CT with 18F-Piflufolastat in PCa Imaging

FDA Clears Dual-Head SPECT/CT System with Deep Learning Image Reconstruction
Latest Videos

CME Content
More News
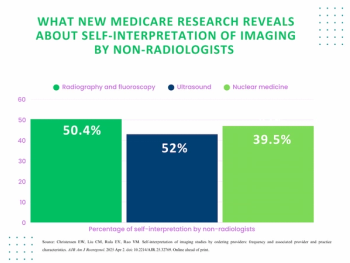
In a review of over 1,630,000 office-based non-breast imaging Medicare fee-for-service (FFS) claims from 2022, researchers found that 39.5 percent of nuclear medicine imaging were interpreted by ordering clinicians.

Catch up on the top radiology content of the past week.
Recent research demonstrated a 59 percent reduced risk of progression or death with the radioligand therapy Pluvicto in comparison to a change of androgen receptor pathway inhibitor (ARPI) for patients with metastatic castration-resistant prostate cancer (mCRPC).
The positron emission tomography myocardial perfusion imaging (PET MPI) agent, which offers a significantly higher half-life than other cardiac PET agents, was recently granted pass-through payment status by CMS that will go into effect on April 1, 2025.
Catch up on the most-well viewed radiology content in March 2025.

Catch up on the most-well viewed prostate imaging content in March 2025.

Catch up on the top radiology content of the past week.
Indicated for use in cases involving suspected metastasis with PCa or suspected PCa recurrence due to elevated PSA level, Gozellix reportedly has a longer shelf life than other gallium-based PET imaging products.

The Reading Room Podcast: Current Perspectives on the Updated Appropriate Use Criteria for Brain PET
In a new podcast, Satoshi Minoshima, M.D., Ph.D., and James Williams, Ph.D., share their insights on the recently updated appropriate use criteria for amyloid PET and tau PET in patients with mild cognitive impairment.

New updates for the cardiac PET-based HeartSee v4.0 software include an emphasis on the evaluation of subendocardial ischemia and border zones.

Catch up on the top radiology content of the past week.
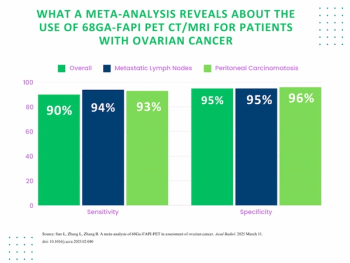
Researchers found that use of 68Ga-FAPI PET was associated with pooled sensitivity of 90 percent for ovarian cancer, according to a recent meta-analysis.

Catch up on the top radiology content of the past week.
Catch up on the most-well viewed prostate imaging content in February 2025.
Catch up on the most-well viewed radiology content in February 2025.

In a recent interview, Abhinav K. Jha, Ph.D., discussed key challenges with the use of SPECT MRI and how an emerging deep learning model may facilitate attenuation compensation without the need for an additional computed tomography (CT) scan.
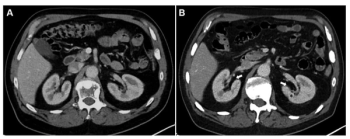
In patients who had at least four cycles of 177Lu-PSMA-I&T for mCRPC, new research shows that a 10 percent or greater decrease in total kidney volume on CT at six months has a 90 percent AUC for predicting estimated glomerular filtration rates (eGFRs) of 30 percent or greater at one year.
The PET radiopharmaceutical SAR-bisPSMA has garnered three FDA fast track designations in a six-month period for use in the detection and management of prostate cancer.
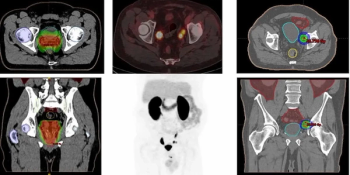
18F-DCFPyL facilitated detection of recurrent prostate cancer in 51 percent of patients with PSA levels ranging between 0.2 to 0.5 ng/ml, according to new research presented at the American Society of Clinical Oncology Genitourinary Cancers (ASCO-GU) Symposium.
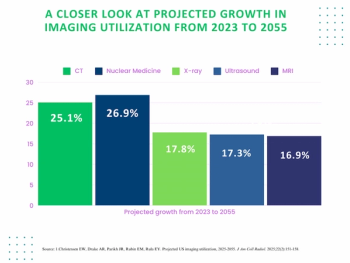
Reviewing current and emerging trends in imaging utilization and the impact of attrition rates and radiology residency positions on the field, researchers explore the future of radiology with two new provocative studies.

Catch up on the top radiology content of the past week.

Phase 2 studies of the BR55 ultrasound contrast agent reportedly demonstrated a 95 percent accuracy in showing the expression of vascular endothelial growth factor receptor 2 (VEGFR2) in bowel segments reflecting active inflammation in patients with Crohn’s disease.

Catch up on the most well-viewed video interviews from Diagnostic Imaging in January 2025.

Catch up on the top radiology content of the past week.
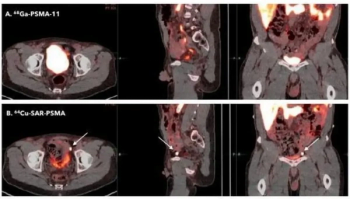
In addition to an August FDA fast track designation for PSMA PET imaging in patients with suspected metastasis, the radiopharmaceutical 64Cu-SAR-bisPSMA has earned another fast track designation for imaging of biochemical recurrence.