
Catch up on the top radiology content of the past week.

Discussing findings from a new study presented at the Society for Breast Imaging (SBI) conference, Shahrzad Tavana, M.D., detailed the significant impact of training sessions for MRI technologists in improving breast positioning, optimal field of view and accuracy of sequence submissions to PACS for breast MRI exams.
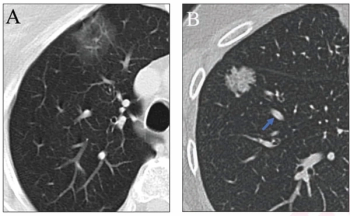
The inclusion of simulated adjudication for resolving discordant nodule classifications in a deep learning model for assessing lung adenocarcinoma on chest CT resulted in a 12 percent increase in sensitivity rate.
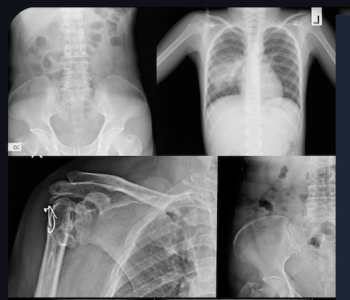
Leveraging artificial intelligence, the Rho software assessment of X-rays may allow earlier detection of bone loss.
Patients with Medicaid or means-tested insurance were over 27 percent more likely to miss mammography appointments, and only 65 percent of women with three of more adverse social determinants of health had a mammography exam in a two-year period covering 2020 and 2021, according to new research and a report from the CDC.
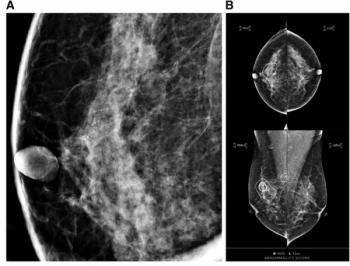
Emerging research suggests that an artificial intelligence (AI) score of 75 or greater for mammography abnormalities more than doubles the likelihood of invasive upgrade of ductal carcinoma in situ (DCIS) diagnosed with percutaneous biopsy.

Review the case and test your knowledge to make the correct diagnosis.
Can consistent timeliness, visual effort and engaging attitude give a radiologist with so-so skills a discernible advantage?
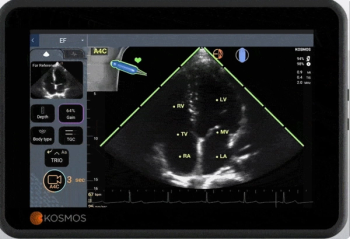
Strain imaging analysis is one of the automated parameters included in the newly FDA-cleared Us2.v2 software for adjunctive review of echocardiographic images.
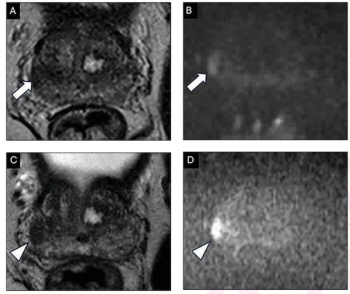
In a recent review, researchers examined the pros and cons of specialized scoring systems for prostate MRI, including the PI-QUAL score, PRECISE recommendations, PI-RR scoring and PI-FAB assessments.

Utilizing PET imaging to assess pathologic complete response to the combination of trastuzumab and pertuzumab for patients with HER2-positive breast cancer, researchers noted a 94.8 percent rate of invasive disease-free survival at three years.

In an attempt to navigate the imbalance between rising imaging volume and a shortage of radiologists, researchers proposed a variety of measures that may provide additional resources, bolster productivity, and help mitigate burnout.

In addition to full-field digital mammography and titanium contrast-enhanced mammography capabilities, the Mammomat B.brilliant system also offers ergonomic, workflow and patient comfort benefits.
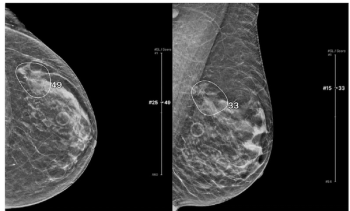
An emerging artificial intelligence (AI) model demonstrated more than 12 percent higher specificity and reduced image reading time by nearly six seconds in comparison to unassisted radiologist interpretation of digital breast tomosynthesis (DBT) images.
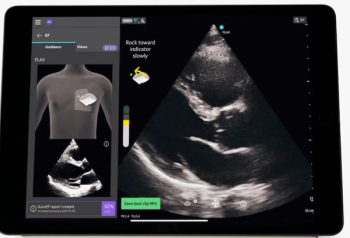
The addition of Caption AI to the handheld ultrasound device Vscan Air SL may facilitate optimal imaging for point-of-care cardiac evaluation.
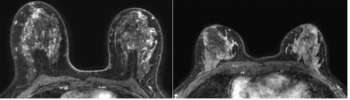
An MRI-based machine learning model demonstrated a comparable background parenchymal enhancement (BPE) hazard ratio to that of manual BPE assessment for breast cancer, according to a study of over 4,500 women with dense breasts.
Up to 48 percent of unnecessary prostate biopsies could be eliminated by limiting biopsy to patients with PI-RADS 4 and higher MRI assessments and a prostate-specific antigen density level below 0.15 ng/mL2, according to a new meta-analysis.
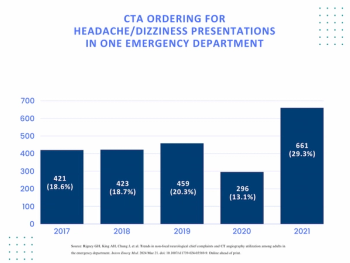
Researchers noted a 67.4 percent increase in head and neck CT angiography and a 38 percent reduction in findings of acute pathology in a recent comparison of 2017 and 2021 statistics for headache and/or dizziness presentations at the emergency department of an urban academic medical center.
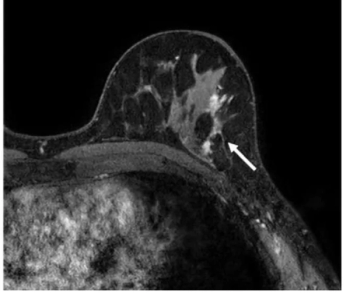
In a new literature review, researchers noted key findings on the use of breast MRI in facilitating breast cancer detection for women with dense breasts and others at high-risk for breast cancer.

Whether you are extolling the benefits of a new radiology gig to a friend or sizing up a potential opportunity based on a colleague’s recommendation, taking the time to make a prudent, objective assessment is usually the best course of action.

Catch up on the top radiology content of the past week.

In a cohort of patients with invasive breast cancer and tumor sizes ranging between 0.3 to 9 cm, image-guided cryoablation was associated with a 10 percent recurrence rate at 16 months, according to research recently presented at the Society of Interventional Radiology (SIR) conference.
Catch up on the most-well viewed radiology content in March 2024.
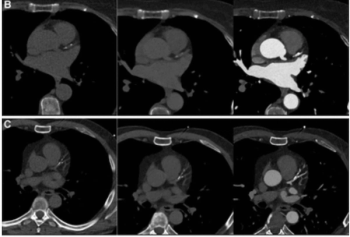
Emerging research on coronary artery calcium scoring for the assessment of coronary artery disease (CAD) suggests the use of virtual non-contrast images from photon-counting CT may lead to a nearly 20 percent reduction in radiation dosing.
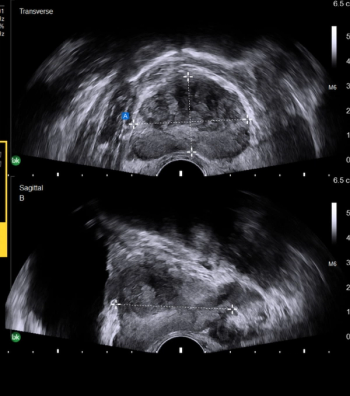
Featuring automated one-click functionality for capturing prostate volume, the AI-enabled software may help shorten workflows for prostate imaging, biopsies, and ultrasound-guided procedures.
Offering improved image clarity capable of capturing details of subtle pathology, the Magnetom Terra.X 7T MRI system reportedly features the first eight-channel parallel transmit architecture for clinical use.
SyMRI 3D reportedly combines precise estimates of regional brain volume with improved resolution bolstering accuracy with lesion analysis.
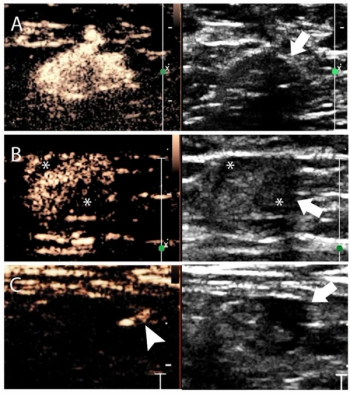
A nomogram that combines contrast-enhanced lymphatic ultrasound and grayscale ultrasound findings had an 88 percent AUC for prediction of three or more metastatic axillary lymph nodes in women with early breast cancer.

The artificial intelligence (AI) modalities CINA-iPE and CINA-ASPECTS may facilitate improved detection of incidental pulmonary embolism and stroke evaluation, respectively, based on computed tomography (CT) scans.