Ultrasound helps to guide percutaneous applications
The number of image-guided percutaneous interventions being performed, including tissue biopsies, fluid aspiration, and catheter insertions, has increased markedly. The rising popularity of these procedures is due to their less invasive nature and lower risk compared with surgery, their high diagnostic accuracy, and the substantial cost savings they provide.
The number of image-guided percutaneous interventions being performed, including tissue biopsies, fluid aspiration, and catheter insertions, has increased markedly. The rising popularity of these procedures is due to their less invasive nature and lower risk compared with surgery, their high diagnostic accuracy, and the substantial cost savings they provide.1-4
Ultrasound enables real-time scanning of the needle from its insertion through the skin to contact with the target. This is a great advantage of using ultrasound for guidance rather than CT or MRI.4 The availability of high-quality ultrasound systems has made it easier to resolve small and/or deep-seated lesions that were previously difficult to visualize. Ultrasound-guided procedures are also a safe, quick, and accurate way of achieving a firm diagnosis or choosing an effective treatment without recourse to laparoscopy or laparotomy.
Practitioners performing ultrasound-guided procedures have a choice of two techniques: freehand and probe-guided.5 Freehand ultrasound requires operators to control the probe with one hand and the biopsy needle with the other. Its chief advantage is the maximal freedom it offers in choosing a needle path. But poor hand-eye coordination can make it difficult to place and identify the needle within the ultrasound imaging plane.
The probe-guided technique uses a needle guide fixed to the ultrasound probe, making it easier for less experienced operators to keep the needle within the imaging plane. The fixed angle between probe and needle limits the selection of needle path, however.5 I recommend using freehand ultrasound for guiding abdominal interventions, despite the long learning curve needed to master the technique.
Percutaneous gastrostomy is a well-established and relatively easy technique. It has a high (up to 99%) technical success rate and a low rate of major complications (2% to 6%).6 Percutaneous gastrostomy can be performed using endoscopic or image-guided methods.7 Gastrostomy under fluoroscopic guidance consists of nasogastric tube insertion, air inflation of the stomach through the nasogastric tube, gastric puncture, guidewire insertion with or without anchoring, and catheter placement.
The critical step is gastric puncture. Gaseous distention of the stomach through the nasogastric tube is essential to avoid major complications, such as transgression of bowel, liver, or vascular structures. Gastric distention makes it easier to penetrate the gastric wall and helps bring the stomach closer to the anterior abdominal wall and displace any interposed bowel.
Ultrasound guidance is useful when tube insertion is not possible; e.g., in patients who have pharyngeal or esophageal stricture, are at risk of tracheal aspiration, and have basal skull fractures. It can also minimize injury to structures adjacent to the fluid-filled stomach. The gas-free collapsed gastric wall has a targetlike appearance on ultrasound; that is, an echogenic center and a hypoechoic periphery (the gastric anterior and posterior walls). The gas-filled gastric wall is usually seen as two layers: an echogenic line with an overlying thin, low-echoic line (anterior stomach wall) (Figure 1A).
The entry site is chosen in the anterior left upper quadrant in the midbody of the stomach. Only the anterior stomach wall should be punctured, with an 18-gauge Seldinger needle. The needle advances to the central echogenic line of the region's targetlike appearance in the collapsed stomach. Once the anterior wall has been punctured, water-soluble contrast can be administered through the needle. The puncture is successful if injected contrast is seen to fill the gastric lumen on fluoroscopy. Gastric distention can then be achieved by injecting air through the needle.
With ultrasound-guided puncture, the needle may pierce the posterior wall. This would cause injected contrast or air to fill the lesser sac. It is very important to demonstrate rugal folds and to see the contrast enter the duodenum.
The steps following successful anterior wall puncture will be easier. A guidewire is passed into the stomach using the Seldinger technique, and the gastrostomy tube is inserted over the guidewire into the gastric lumen. Tube displacement should be ruled out by demonstration of the gastric rugal folds and the passage of contrast into the duodenum through the gastrostomy tube (Figure 1B).
Elective jejunostomy has traditionally been a surgical procedure, though less invasive ways of performing this procedure have been tried using laparoscopy, gastrointestinal endoscopy, and fluoroscopically guided percutaneous catheterization.8 Jejunostomy has been performed mainly for the enteral administration of food. Other indications include the diversion of succus from a leaking anastomosis after esophagectomy and access to a limb of a Roux-en-Y anastomosis to facilitate biliary interventions.
Percutaneous jejunostomy for enteral feeding is not widely accepted due to the simpler access offered by gastronomy. The procedure has been reserved for patients whose stomachs have been removed or are inaccessible.8 Problems include difficulties identifying the jejunal lumen radiologically in patients with obstructions to the upper digestive tract, compliance of the jejunal wall, and jejunum mobility.8 Ultrasound guidance can make it easier to catheterize the jejunal loop. The jejunum resembles a target and appears as a 2 to 3-cm echogenic structure on axial ultrasound images acquired with a linear-array, 5 to 12-MHz probe. Peristalsis and, occasionally, a fluid-filled lumen is observed in the upper abdomen.
Bowel distention and jejunopexy are two important requirements for reliable percutaneous jejunostomy.8 Hypotonic agents, such as glucagons, or anticholinergic drugs may help to reduce jejunum mobility. Administration of water through the nasogastric tube enhances bowel distention and improves the sonographic visualization of targets.
The target jejunum is selected on ultrasound, then the bowel is punctured using an 18-gauge Seldinger needle. Successful puncture is confirmed on fluoroscopy after contrast injection through the needle. A suture anchor is loaded in the needle and pushed into the jejunum using a guidewire. This anchoring is important because it fixes the jejunum to the anterior abdominal wall and prevents pericatheter leakage. The tract is dilated and a 6 to 10-Fr catheter is inserted over the guidewire, in a method similar to that used in gastrostomy. The technical success and complication rates of percutaneous jejunostomy under ultrasound guidance compare well with those for surgery and endoscopy (Figure 2).8
HEPATIC APPLICATIONS
Image-guided catheterization of liver abscesses is a routine procedure. Abscess puncture is performed under ultrasound guidance, while insertion of the guidewire and catheter are done under fluoroscopic guidance.
Percutaneous catheterization can be guided by ultrasound alone. This is useful when patients cannot move to the fluoroscopy room, portable fluoroscopy is not available, or the use of ionizing radiation is to be avoided. The guidewire is visualized on ultrasound as an echogenic line. Injection of a small amount of air or fluid may help identify the catheter's location if it cannot be seen on ultrasound. Air will produce ring-down artifacts and shadowing, and the fluid will produce a flow-jet.
Ultrasound guidance during percutaneous transhepatic biliary drainage also offers several advantages. Fewer needle passes are needed to cannulate the bile duct. Practitioners can select which duct they use in patients who have hepatic masses adjacent to potential drainage routes or strictures involving multiple sites of the biliary tree. Prompt detection of complications-e.g., perihepatic hematoma near the intraperitoneal entering site, or intrahepatic hematoma or biloma around the catheter placed in the bile duct-is possible as well.
Ultrasound-guided liver biopsy is a safe and accurate method widely accepted for the diagnosis of hepatic focal lesions and diffuse liver disease.
FURTHER BIOPSIES
Many abdominal organs beyond the liver can be targets for ultrasound-guided core biopsy as well. The freehand technique is essential, enabling operators to visualize the target lesion perfectly, choose a proper and safe biopsy route, and evaluate vessels, organs, and portions of bowel adjacent to the target comprehensively.
All solid abdominal organs can be targeted by ultrasound-guided biopsy. Focal lesions in the spleen and adrenal glands, and even retroperitoneal masses or para-aortic lymphadenopathies, can be candidates for percutaneous biopsy. This depends, however, on operators ensuring that the biopsy needle follows the correct path.9,10
Thick-walled bowel segments and bowel-related masses can be biopsied under ultrasound guidance too. These are visualized easily as having a target-like or pseudokidney appearance.11 A diagnostic biopsy of the bowel, using an 18-gauge cutting needle, can be performed safely with ultrasound guidance to acquire a satisfactory sample for histological examination.11
Omental pathology includes primary and secondary neoplastic conditions and inflammatory and vascular diseases. These are seen as omental masses, omental infiltration, peritoneal thickening, or ascites on ultrasound and contrast-enhanced CT. Sampling errors remain a problem for core biopsy. Ultrasound-guided core biopsy of omental masses, however, is a safe and accurate way to reach a firm diagnosis without recourse to laparoscopy or laparotomy.12 Core biopsy results from an omental mass can provide a firm indication of the best treatment strategy and the likely prognosis.
Several diseases, such as tuberculosis, carcinomatosis peritonei, and lymphoma, have unusual presentations on CT and ultrasound. They appear as omental infiltration alone on contrast-enhanced CT and as omental thickening on ultrasound. No mass is seen in either case. It is difficult to determine the likely safety of core biopsy, the possibility of adequate sampling, and the best site to sample in these cases.
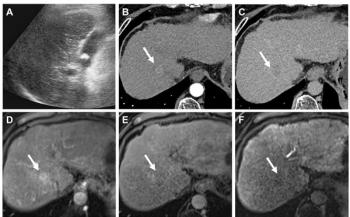
Ultrasound-guided core biopsy of the thickened omentum, using 18-gauge cutting needles, is a safe and accurate way of obtaining adequate tissue for a firm diagnosis of omental pathology. The yield from ultrasound-guided core biopsy of omental infiltration on contrast-enhanced CT may increase if the target is the low-echoic area (where present) in the thickened omentum, which is echogenic on ultrasound. Ultrasound-guided core biopsy can replace laparoscopic biopsy or laparotomy only when differentiating between carcinomatosis peritonei and inflammatory processes such as tuberculosis (Figure 3).
Laparotomy, which is only for diagnosis and not for treatment, can be completely replaced by ultrasound-guided interventional procedures. Success with these procedures, however, depends on practitioners visualizing the targets completely.
References
- Bret PM, Fond A, Casola G, et al. Abdominal lesions: a prospective study of clinical efficacy of percutaneous fine-needle biopsy. Radiology 1986;159(2):345-346.
- Hopper KD. Percutaneous, radiographically guided biopsy: a history. Radiology 1995;196(2):329-333.
- Dodd GD 3rd, Esola CC, Memel DS, et al. Sonography: the undiscovered jewel of interventional radiology. Radiographics 1996;16(6):1271-1288.
- Sheafor DH, Paulson EK, Simmons CM, et al. Abdominal percutaneous interventional procedures: comparison of CT and US guidance. Radiology 1998;207(3):705-710.
- Phal PM, Brooks DM, Wolfe R. Sonographically guided biopsy of focal lesions: a comparison of freehand and probe-guided techniques using a phantom. AJR 2005;184(5):1652-1656.
- van Overhagen H, Ludviksson MA, Lameris JS, et al. US and fluoroscopic-guided percutaneous jejunostomy: experience in 49 patients. J Vasc Interv Radiol 2000;11(1):101-106.
- Gore RM, Levine MS, Laufer I. Textbook of gastrointestinal radiology. Philadelphia: Saunders, 1994:336-340.
- Cope C, Davis AG, Baum RA, et al. Direct percutaneous jejunostomy: techniques and applications-ten years experience. Radiology 1998;209(3):747-754.
- Zerem E, Bergsland J. Ultrasound guided percutaneous treatment for splenic abscesses. The significance in treatment of critically ill patients. World J Gastroenterol 2006;12(45):7341-7345.
- Nagano T, Nakai Y, Taniguchi F, et al. Diagnosis of paraaortic and pelvic lymph node metastasis of gynecologic malignant tumors by ultrasound-guided percutaneous fine-needle aspiration biopsy. Cancer 1991;68(12):2571-2574.
- Tudor GR, Rodgers PM, West KP. Bowel lesions: percutaneous US-guided 18-gauge needle biopsy-preliminary experience. Radiology 1999;212(2):594-597.
- Pombo F, Rodriguez E, Martin R, et al. CT guided core-needle biopsy in omental pathology. Acta Radiol 1997;38(6):978-981.
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.