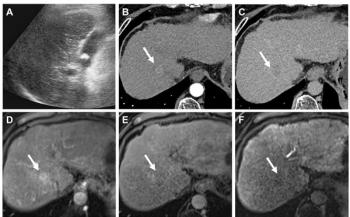
FDA warning stalls AIUM-supported ultrasound contrast trial
Plans for a clinical trial designed to overcome long-standing regulatory obstacles to the general clinical use of ultrasound contrast media in the U.S. have been knocked off track by an FDA-mandated black box safety warning for two microbubble agents approved for echocardiography.
Plans for a clinical trial designed to overcome long-standing regulatory obstacles to the general clinical use of ultrasound contrast media in the U.S. have been knocked off track by an FDA-mandated black box safety warning for two microbubble agents approved for echocardiography.The implications of black box labeling for Bristol-Myers Squibb's Definity and GE Healthcare's Optison agents were clarified Nov. 6 during a teleconference involving the representatives of the American Institute of Ultrasound in Medicine and the FDA Division of Medical Imaging and Radiopharmaceutical Drug Products. The FDA and AIUM have been engaged in talks since March 2006 on how to secure clearance for clinical indications of microbubble-based ultrasound contrast agents outside the heart, according to Dr. Lennard Greenbaum, immediate past president of AIUM. The agents have been in general use in Canada and Europe for several years. Ultrasound advocates consider access to contrast media crucial to the continued clinical acceptance of the modality in the U.S. Diagnostic ultrasound has lost ground since the mid-1990s to MRI and CT, both of which use contrast media. Most investigative ultrasound probes designed for future molecular imaging applications of the modality are based on the same lipid microsphere technologies that have failed to gain FDA approval for general radiological use, said Dr. Katherine Ferrara, chair of biomedical engineering at the University of California, Davis. "What is at stake here is ultrasound contrast agents ever being used for a noncardiac indication," Greenbaum said in an interview with Diagnostic Imaging. "Continued regulatory delay deprives patients of a meaningful diagnostic tool." FDA representatives had expressed general support for a draft plan from the AIUM in November 2006 for a clinical trial testing the efficacy of microbubble contrast for transarterial chemoembolism of liver lesions, Greenbaum said. FDA officials, including Dr. George Mills, the then-director of the Division of Medical Imaging and Radiopharmaceutical Research, suggested that the AIUM group use an approved cardiac agent (Definity or Optison) in its trial, Greenbaum said. Mills resigned from the FDA in February.The FDA's black box order issued Oct. 15 followed reports of 11 deaths associated with Definity and Optison. According to the order, four of those deaths were caused by cardiac arrest that occurred either during infusion or within 30 minutes after infusion of a microbubble ultrasound agent. The black box warning emphasizes the risk for serious cardiopulmonary reactions. It establishes a contraindication for patients with unstable cardiopulmonary status, the group that many cardiologist say are most likely to be prescribed Definity or Optison."The black box ruling came as a total surprise,", Greenbaum said.It would handicap the AIUM's clinical trial initiative, he said.Participants in the FDA teleconference from the AIUM, in addition to Greenbaum, included:
- AIUM president Dr. Carmen Valente
- AIUM president-elect Dr. Harvey Nessenbaum
- Canadian ultrasound contrast researcher Dr. Stephanie Wilson
- University of Michigan biomedical engineer J. Brian Folkes, Ph.D.
Several FDA representatives, including Dr. Ira Krefting and Dr. Louis Marzella from the Division of Medical Imaging and Radiopharmaceutical Drug Products, also participated.
FDA officials acknowledged during the teleconference a lack of corresponding adverse reactions to microbubble contrast when administered for noncardiac indications, Greenbaum said. Its better safety history may stem from the relative lack of serious comorbidity among noncardiac patients compared with cardiac patients. Dose differences may also factor in.Still, FDA officials turned down a request during the teleconference to waive its requirement for the black box warnings on microbubble contrast media to be used in the proposed AIUM trial. It also held to a rule requiring close patient monitoring in the 30 minutes following contrast administration."Some radiologists tell me there may be difficulty getting an institutional review board to approve a project associated with such a severe warning," Greenbaum said. "Radiology departments are also not set up to do extended patient monitoring."The AIUM project was on hold as of Nov. 20. Radiologists associated with the trial have communicated with cardiologists who oppose the black box order. Dr. Michael L. Main, medical director of the electrocardiography lab at the Mid America Heart Institute, for example, was joined by 160 cosigners, including cardiologists and radiologists, in a Nov. 10 letter to the FDA expressing concern about the black box warning. In the letter, Main argued that four patient deaths may have been caused by underlying cardiovascular disease. He also said that the deaths occurred over a six-year period while about two million doses of the ultrasound contrast media were administered. The data suggest an event rate for ultrasound microbubble contrast of one in 500,000 cases, which Main said compares well against the one in 2500 risk of cardiac death from exercise treadmill testing.The AIUM may also become directly involved in an investigation of the deaths that led to the black box warnings. All deaths associated with ultrasound contrast occurred outside the U.S. Data describing the circumstances of those deaths reside with Bristol-Myers Squibb and GE, Greenbaum said. A multispecialty group is considering whether to pursue the investigation of the deaths independently or in conjunction with the FDA. The bioeffects committee of the AIUM has announced that it cannot comment on the FDA statements concerning the safety of ultrasound contrast unless it can review that data, Greenberg said. "Unless there is a review of the data, we cannot expect a modification or removal of the black box (warning)," he said. For more information from the Diagnostic Imaging archives:
Barnes-Jewish drops echo contrast before FDA alert
Black box warning planned for ultrasound microbubble contrast
AIUM white paper targets FDA's contrast ultrasound approval
Ultrasound's future in play: Will radiologists remain in the picture?
Newsletter
Stay at the forefront of radiology with the Diagnostic Imaging newsletter, delivering the latest news, clinical insights, and imaging advancements for today’s radiologists.