AI Coverage
about 6 hours ago
Diagnostic Imaging's Weekly Scan: February 1 — February 7about 11 hours ago
Radiology Roundup of New FDA Clearances — February 1 — February 7Latest News

Diagnostic Imaging's Weekly Scan: February 1 — February 7
Radiology Roundup of New FDA Clearances — February 1 — February 7
FDA Clears AI-Powered Triage Platform for Digital Breast Tomosynthesis

Is AI Better Than Neuroradiologists at Evaluating Aneurysm Growth on CTA and MRA Scans?
FDA Clears 3T MRI Device for Neonates and Infants

Shorts










Podcasts
Videos
Continuing Medical Education
All News

Wendie Berg, M.D., Stamatia Destounis, M.D., and Amy Patel, M.D., share their thoughts and perspectives on key findings from the Lancet mammography study on AI and interval breast cancer, and how they have incorporated AI into their practices.

A high SUVmean on PSMA PET imaging was associated with 95 more days of mean progression-free survival (PFS) in contrast to those with a low median SUVmean, according to a new study of patients with mCRPC treated with (225Ac)Ac-PSMA-I&T.

The CT-based RevealAI-Lung software reportedly offers a Malignancy Similarity Index score to facilitate evaluation of incidental lung nodules.

In addition to significantly reduced radiation dosing in comparison to energy-integrating detector CT (EID-CT), photon-counting CT provided higher detection of enhancement-related malignant features, according to new prospective research involving 200 patients with lung cancer.

The Brainomix 360 Stroke Next Generation software reportedly includes automated assessment of net water uptake (NWU), a CT-based biomarker that may improve risk stratification for patients with severe strokes.

In a recent interview, Amy Patel, M.D., discussed key targets in legislation for breast imaging in 2026 and offered advice for breast radiologists seeking to get more involved in advocacy.
The Allia Moveo C-arm platform reportedly offers ergonomic maneuverability, enhanced artifact reduction and flexibility with the use of cone-beam CT.

Are non-physicians playing around with your radiology reporting macros?

Catch up on the top radiology content of the past week.

In a comparative study involving over 105,000 women, researchers found the use of adjunctive AI for mammography triage resulted in a 16 percent lower rate of invasive interval cancers in comparison to double reading by radiologists without AI.

The Biograph One PET/MRI system may facilitate accelerated scan times, improved visualization and more efficient assessments of theranostic approaches for patients with oncologic and neurological diseases.

In a new podcast episode, Kernesha Weatherly, DHA, emphasized the importance of communication with radiologists, ordering providers and patients to identify challenges with follow-up of incidental imaging findings, and how AI helped bolster data and “line of sight” in these cases at Ochsner Health.

Catch up on the most-well viewed radiology content in January 2026.

Employing AI-powered automation, the MIM LesionID™ Pro reportedly bolsters the efficiency of whole-body tumor burden analysis with PSMA PET/CT and SPECT/CT.
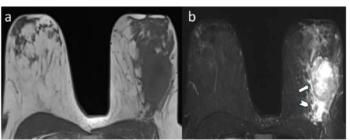
For young women with breast cancer, peritumoral edema on pre-op breast MRI was associated with over a 3.6-fold higher likelihood for reduced disease-free survival, according to new research.